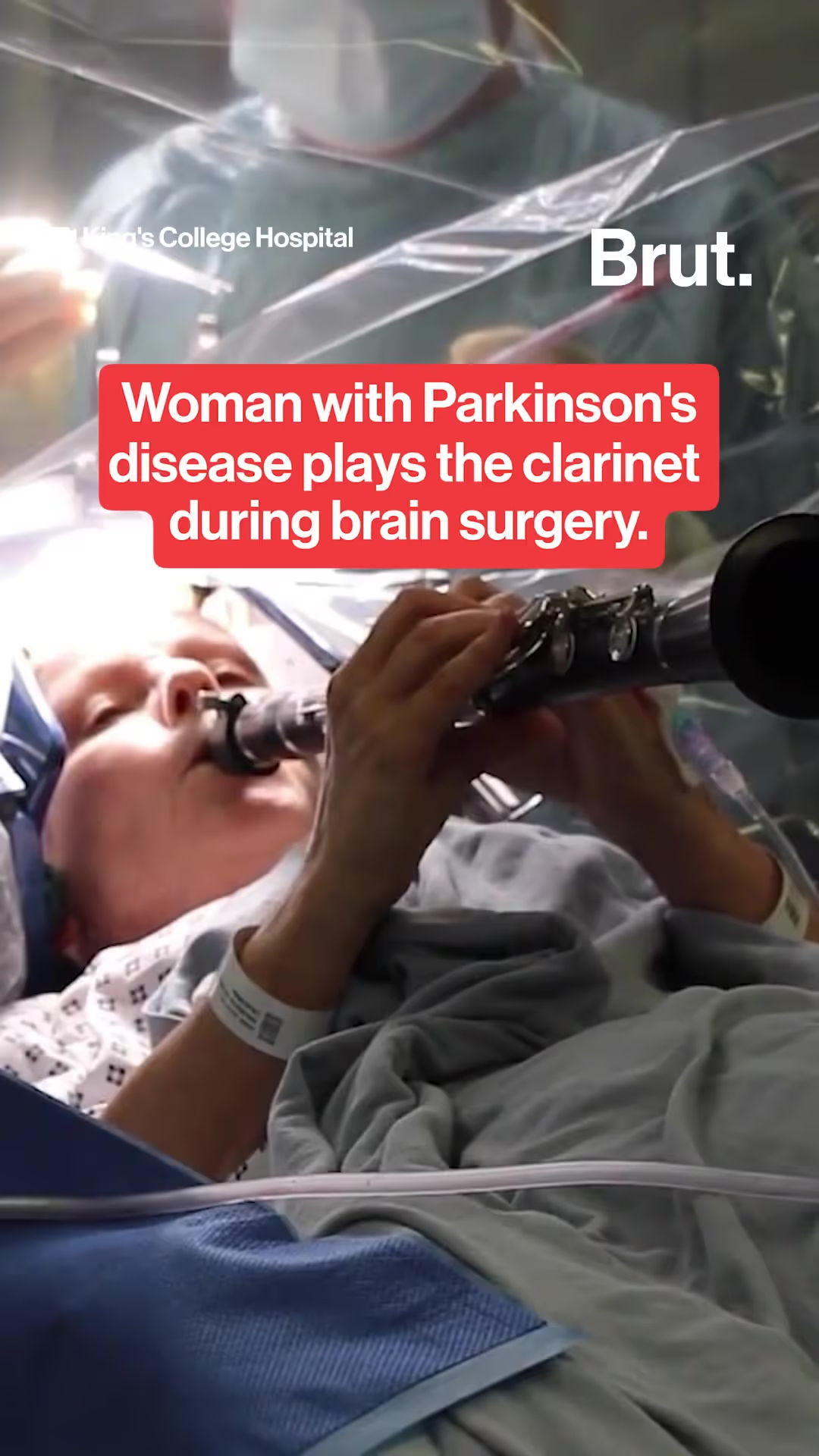
The first trials of coronavirus vaccine
Vaccine trials for coronavirus
The US started vaccine trials for COVID-19.
Jennifer Haller, a healthy volunteer, was injected with a dose of an experimental vaccine as part of a clinical trial in Seattle. “Everybody is feeling so helpless right now, and I realised that there was something that I could do to help. I'm excited to be here.”, she told Brut. Each participant of the study will receive two different injections a month a part that varies in dose amounts.
45 patients are expected to participate
The vaccine was fast-tracked into clinical trial without thorough animal testing which is usually strictly required. Dr. Lisa Jackson, a study investigator at Kaiser Permanente, shared, “These are people who are volunteering to be in a trial for a vaccine that's never been given to humans before. And so, we take all the precautions we can take. But there's some inherent risks there because we can't know what we haven't evaluated yet. So, yes, they're... I think that most of them or all of them are acting out of a sense of public good, and wanting to help in this process.”
The vaccine seeks to replicate proteins similar to the virus' to spark an immune response
Dr. Jackson continues, “There's no chance of getting coronavirus from the vaccine. The vaccine is not made from the virus. It does not have any part of the virus. It includes a genetic code that instructs the cells in the body to make a protein that the virus has in order to induce an immune response against that protein, which would hopefully allow an immune response to be built more quickly, should a person later become infected with the actual virus.” Even though it has jumped through some administrative hoops the vaccine may only be available for public use in 12-18 months.
Brut.
The first trials of coronavirus vaccine
Vaccine trials for coronavirus
The US started vaccine trials for COVID-19.
Jennifer Haller, a healthy volunteer, was injected with a dose of an experimental vaccine as part of a clinical trial in Seattle. “Everybody is feeling so helpless right now, and I realised that there was something that I could do to help. I'm excited to be here.”, she told Brut. Each participant of the study will receive two different injections a month a part that varies in dose amounts.
45 patients are expected to participate
The vaccine was fast-tracked into clinical trial without thorough animal testing which is usually strictly required. Dr. Lisa Jackson, a study investigator at Kaiser Permanente, shared, “These are people who are volunteering to be in a trial for a vaccine that's never been given to humans before. And so, we take all the precautions we can take. But there's some inherent risks there because we can't know what we haven't evaluated yet. So, yes, they're... I think that most of them or all of them are acting out of a sense of public good, and wanting to help in this process.”
The vaccine seeks to replicate proteins similar to the virus' to spark an immune response
Dr. Jackson continues, “There's no chance of getting coronavirus from the vaccine. The vaccine is not made from the virus. It does not have any part of the virus. It includes a genetic code that instructs the cells in the body to make a protein that the virus has in order to induce an immune response against that protein, which would hopefully allow an immune response to be built more quickly, should a person later become infected with the actual virus.” Even though it has jumped through some administrative hoops the vaccine may only be available for public use in 12-18 months.
Brut.